Abstract
A 43 yo Caucasian woman, previously healthy, nulliparous, no family history, and on no medications, had a normal primary care checkup, and routine labs including Hgb 12.2 gm/dL and platelets (plts) 124 K/uL (normal 179-408), bilirubin (bili) 0.7 (normal <1.3) mg/dL.
Six days later, she presented with weakness, anorexia, nausea, vomiting, and vaginal bleeding. There was vague abdominal tenderness, no bleeding per rectum, ecchymoses, and petechiae, and mild lethargy.
Hgb was 8.8 gm/dL, WBC 7,500 K/uL, and plts 17 K/uL. Peripheral blood smear demonstrated thrombocytopenia and >20 schistocytes per oil immersion field. Serum haptoglobin was <14 (normal 30-200) mg/dL.
Serum electrolytes, liver, and renal function were normal. Total bili was 5.0 mg/dL, direct bili was 0.4 mg/dL. Lactate dehydrogenase (LDH) was 6032 (normal 313-618) U/L. Serum lipase was 746 (normal 23-300) U/L.
The fibrinogen was 322 (normal 165-432) mg/dL. The D-Dimer was 3781 (normal <500) ng/dL.
A stool specimen PCR test did not detect DNA or RNA from 31 pathogenic/toxigenic bacteria, parasites, nor viruses.
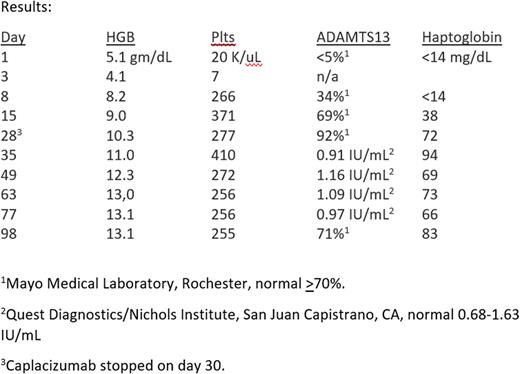
A Disintegrin And Metalloprotease with a ThromboSpondin type 1 motif, member 13 (ADAMTS13) level on presentation was <5% (normal > 70%) and the inhibitor was negative (Mayo Medical Laboratories, Rochester).
A diagnosis of thrombotic thrombocytopenia (TTP) was made and plasmapheresis with plasma exchange using fresh frozen plasma (PEX) was recommended. The patient and family refused, due to religious convictions (Jehovah Witness) against receiving "any of the 4 blood products": RBC, WBC, platelets, or plasma.
She did agree to treatment according to Chander, et al, NEJM 381(1) 2019: pp 92-94: caplacizumab 11 mg subcutaneously daily x 30 days, prednisone 1 mg/kg x 14 days, then tapered over 7 days, rituximab 375 mg/m2 day 1, 8, 15, and 22, and Koate-DVI (a human plasma derived factor VIII concentrate that contains ADAMTS13) 3 days a week for 2 weeks.
This treatment was well tolerated, with no unusual or unexpected toxicities, except for minor bruising around the SQ caplacizumab injection sites. Clinical results are noted below.
This previously healthy woman had sudden onset of severe TTP. Her clinical outcome - without PEX - was dramatically successful, clearly lifesaving. Improvement was evident in several days, and she has enjoyed a complete and - so far - durable remission, with minimal toxicity. She is being maintained on low dose aspirin and followed carefully for relapse.
TTP is caused by depletion of ADAMTS13, leading to accumulation of very large vWF multimers, which causes thrombotic microangiopathy from platelet aggregation and consumption. Caplacizumab targets the A1 domain of von Willebrand factor (VWF), thereby blocking platelet binding, and has resulted in improved outcomes, when added to traditional plasma exchange and immunosuppressive therapy.
There are 3 published reports of treating TTP with caplacizumab and immunosuppression without PEX describing 3 successful patient outcomes: Chander, et al, NEJM 381(1) 2019: 92-94, Chai, et al, J Clin Apher 2015: 46-9, and Sukumar, et al, Am J Hematol 2020, 95: E76-77. I received personal communication from Drs. James George and Spero Cataland that additional successful outcomes have occurred under their care and elsewhere, and that clinical trials using this approach are being contemplated. (Their help in this patient's management and in the review of this manuscript are acknowledged and appreciated.)
The purpose of this abstract is to add to the limited published experience using caplacizumab without plasma exchange in patients with TTP.
Disclosures
No relevant conflicts of interest to declare.
Author notes
*Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal